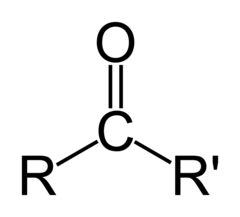
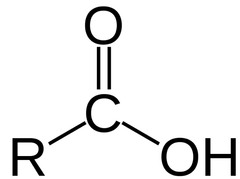
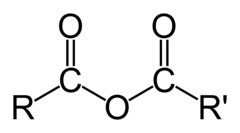
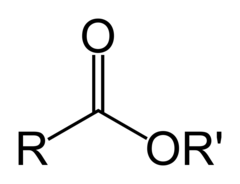
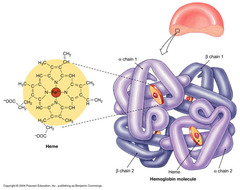
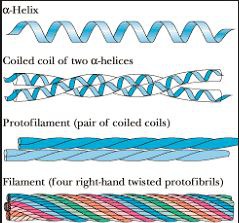
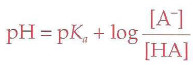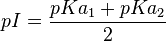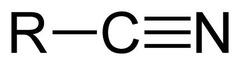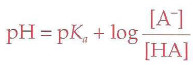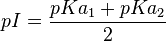The most abundant molecule in the human body is...
Water
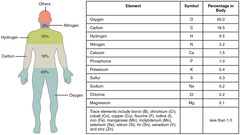
What are the four most abundant elements in a human body?
Carbon, Nitrogen, Oxygen, Hydrogen
Which three cellular components are present in both prokaryotes and eukaryotes?
A. ribosomes, chloroplasts, mitochondria
B. nucleus, ribosomes, RNA
C. RNA, DNA, ribosomes
D. endoplasmic reticulum, DNA, RNA
E. mitochondria, DNA, RNA
C. RNA, DNA, ribosomes
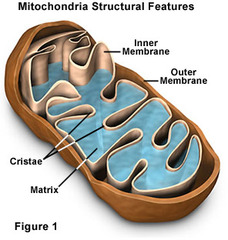
The bulk of aerobic metabolism in eukaryotic cells takes place in the...
Mitochondria
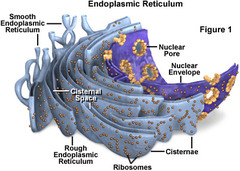
Which cellular compartment or organelle is involved in the synthesis of proteins and lipids?
Endoplasmic Reticulum
True or False? Carbon, nitrogen, oxygen, hydrogen and calcium are the five most prevalent elements found in the human body.
True
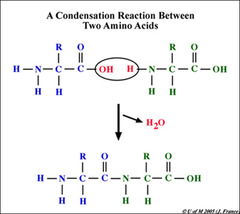
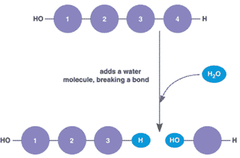
Condensation Reaction
A chemical reaction in which two or more molecules combine to produce water and another simple molecule
Hydrolysis Reaction
A chemical reaction that breaks apart a larger molecule by adding a molecule of water
Brønsted-Lowry Acid
Proton donor
Brønsted-Lowry Base
Proton acceptor
Henderson-Hasselbalch Equation
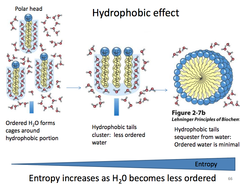
Hydrophobic Effect
the observed tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules; occurs because interactions between the hydrophobic molecules allow water molecules to bond more freely, increasing the entropy of the system and decreasing the surface area of the non polar molecules
Amphiphilic / Amphipathic
Of or relating to a molecule having a polar, water-soluble group attached to a nonpolar, water-insoluble hydrocarbon chain.
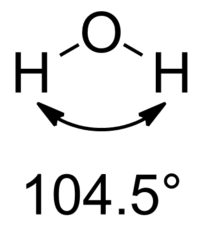
The angle of H2O is...
104.5º
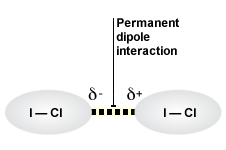
Interactions Between Permanent Dipoles
interspecific interactions
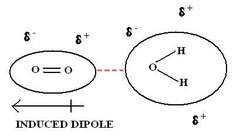
Dipole-Induced Dipole Interactions
dipole moment of polar molecule and polarizability of non-polar molecule
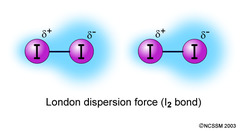
London Dispersion Forces
Occur between two instantaneous dipoles; the weakest dipole-dipole force (vs. hydrogen bonds which are the strongest dipole-dipole forces).
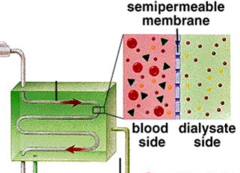
Dialysis
a procedure to remove waste products from the blood of patients whose kidneys no longer function; blood taken out of the body and urea and creatinine are removed from the blood by diffusion
Buffer
A solution that minimizes changes in pH when extraneous acids or bases are added to the solution; pH and pka must be within 1 of each other to be effective
Good's Buffers
1. pka from 6-8
2. Soluble in water
3. Membrane impermeability
4. Minimal salt effect (won't interact with other ions)
5. Minimal effect from temp or concentration
6. Well defined or non existent mineral cations
7. Biochemically inert (won't break down)
8. Chemical stability
9. Optical absorbance (λ < 230 nm)
10. Ease of preparation
The lower the pka, the _____ (stronger/weaker) the acid
The lower the pka, the stronger the acid
The lower the pka, the ________ (more/less) H+ is dissociated
The lower the pka, the MORE H+ is dissociated
As pka increases, molecules tend to ____ (lose / gain) acidic hydrogen ions
As pka increases, molecules tend to lose acidic hydrogen ions
Metabolic Acidosis
Characterized by a DECREASE in HCO₃⁻ level in the serum, leading to an increase in H⁺ concentration and a decrease in pH (below 7.35), , and hyperventilation and a resulting drop in CO2 levels.
Metabolic Alkalosis
Characterized by an INCREASE in the HCO₃⁻ levels in the serum, leading to a decrease in the H⁺ concentration and an increase in the pH (increase above 7.45), and hypoventilation (too little breathing) and a resulting accumulation of CO2.
Respiratory Acidosis
Characterized by excessive RETENTION of CO2 due to hypoventilation; therefore, an increased HCO₃⁻ concentration produces an increase in H+ ions and a decrease in the pH (decreases below 7.35).
Respiratory Alkalosis
Characterized by a decrease in HCO₃⁻ level in the serum, leading to an increase in H⁺ concentration and an increase in pH (above 7.35), , and hyperventilation and a resulting drop in CO2 levels.
A red blood cell has an internal salt concentration of ~150mM. The cell is placed in a beaker of 500mM salt. Assuming that the cell membrane is permeable to water but not to ions, water will move from the _____ (lower / higher) field with incorrect answer salt concentration to the _________ (lower / higher) field with incorrect answer salt concentration. If the membrane were permeable to ions, the solutes would diffuse _____ (into / out of) field with correct answer the cell
A red blood cell has an internal salt concentration of ~150mM. The cell is placed in a beaker of 500mM salt. Assuming that the cell membrane is permeable to water but not to ions, water will move from the HIGHER field with incorrect answer salt concentration to the LOWER field with incorrect answer salt concentration. If the membrane were permeable to ions, the solutes would diffuse INTO field with correct answer the cell
Which of the following correctly lists bonding interactions in terms of their increasing strength:
A) Hydrogen bonds < ionic interactions < covalent interactions < Van der Waals interactions
B) Covalent bonds < hydrogen bonds < ionic interactions < Van der Waals interactions
C) Van der Waals interactions < hydrogen bonding < ionic interactions < covalent bonds
D) Van der Waals interactions < ionic interactions < hydrogen bonding < covalent bonds
C) Van der Waals interactions < hydrogen bonding < ionic interactions < covalent bonds
Which of the following statements about water is FALSE?
A) Substances with polar functional groups are generally soluble in water.
B) The hydrophobic effect is driven largely by the entropy of the non-polar substance.
C) Water will act as a hydrogen bond acceptor with hydroxyl groups.
D) Water carries a dipole because of the geometry of the two oxygen-hydrogen bonds.
E) A network of electrostatic interactions exists between the molecules in water.
B) The hydrophobic effect is driven largely by the entropy of the non-polar substance.
The insolubility of nonpolar molecules in water is due to the large _______, which is the result of water molecules forming an ordered network surrounding nonpolar molecules.
Negative entropy
Osmotic pressure is a function of
A) humidity
B) solute size
C) solute concentration
D) van der Waals forces
E) solute vapor pressure
C) solute concentration
The pH of coffee is 5.6. The pH of grapefruit juice is 2.6. This means that the proton concentration in coffee is
A) 3000 times higher than in grapefruit juice
B) 3000 times lower than in grapefruit juice
C) a thousand times higher than in grapefruit juice
D) a thousand times lower than in grapefruit juice
E) 3 times the proton concentration of grapefruit juice
D) a thousand times lower than in grapefruit juice.
Open System
A system in which both energy and matter are exchanged with the surroundings.
Closed System
A system in which energy, but not matter, is exchanged with its surroundings.
Isolated System
A system that exchanges neither matter nor energy with its surroundings.
Living organisms are _____ systems. Why?
Open systems because they take nutrients, release waste, and generate work and heat
Avogadro's Number
6.02 x 10^23
First Law of Thermodynamics
Energy can be transferred and transformed, but it cannot be created or destroyed.
q = heat absorbed ____ system ____ surroundings
q = heat absorbed BY system FROM surroundings
w = work done ____ system ____ surroundings
w = work dome ON system BY surroundings
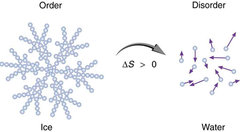
Explain what happens to water as it freezes
As water cools towards 4ºC, it actually becomes more dense. Then as it reaches 0ºC, its density decreases as its structure opens with freezing. This allows the frozen water / ice to float in water.
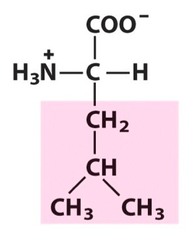
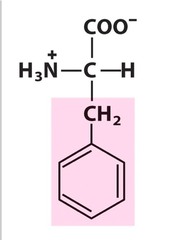
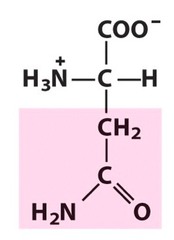
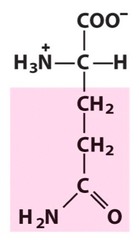
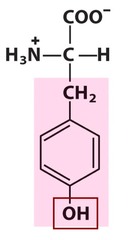
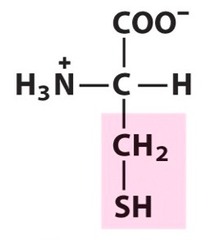
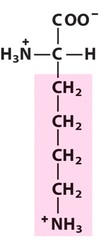
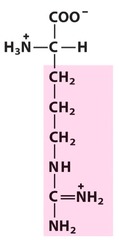
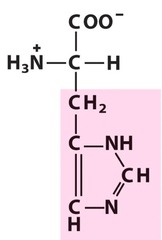
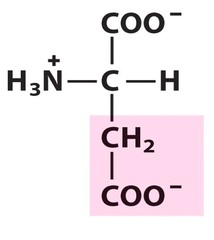
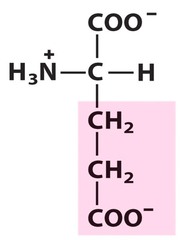
Amino Acid F
Phenylalanine
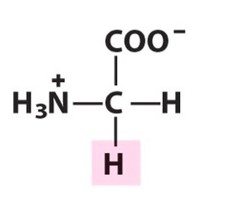
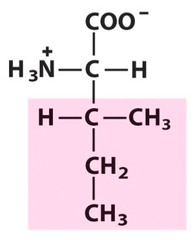
Amino Acid D
Aspartic acid
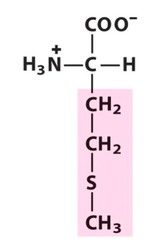
Amino Acid E
Glutamic acid
Enthalpy (H) Equation
H = U + PV
Hess's Law
Regardless of the multiple stages or steps of a reaction, the total enthalpy change for the reaction is the sum of all changes
Second Law of Thermodynamics
says that the entropy (disorder) of the universe is increasing. One corollary of the Second Law of thermodynamics is the concept that, in most energy transformations, a significant fraction of energy is lost to the universe as heat.
Entropy (S)
a measure of the number of specific ways in which a thermodynamic system can be arranged, commonly known as disorder
In a constant energy process (ΔU = 0) ΔS > 0
Gibbs Free Energy
the change in free energy for a process, combines enthalpy, entropy, and temperature
Negative - spontaneous
= 0 process at equilibrium
Conversion from C° to K
K = C° + 273.15
ΔG° Equation
ΔG° = -RT ln Keq
R = 8.314 J/mol•K
Concentration of water according to biochemists
55.5M
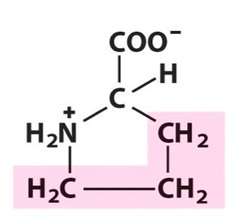
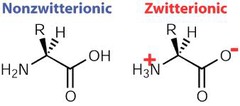
Zwitterions
molecules with both a positive and negative charge that cancel each other out
When pH < pka the molecule is...
PROTINATED
When pH > pka the molecule is...
UNPROTINATED
Isoelectric Point
When the molecule is electrically neutral, pI=pH
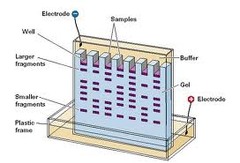
Electrophoresis
The movement of suspended particles through a fluid or gel under the action of an electromotive force applied to electrodes in contact with the suspension.
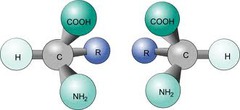
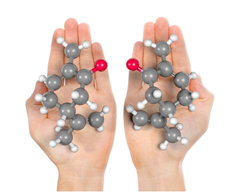
Chirality
If an objects mirror image cannot be superimposed on the original object; lacks internal plane of symmetry
Enantiomers
Non-superimposable mirror image
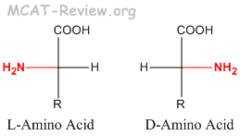
Amino acid residues of all proteins have the __ configuration
L configuration
Essential amino acids
Amino acids that are needed, but cannot be made by the body; they must be eaten in foods
Phe, Val, Thr, Trp, Ile, Met, His, Leu, Lys
PVT TIM HaLL
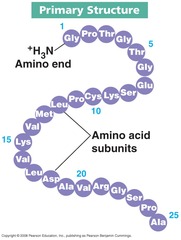
Primary Structure of Protein
Amino acid sequence of its polypeptide chains
For a protein of n residues there are ___ possible sequences
20^n
Neutral Drift
Random mutations in time that do not affect function
Invariant Residues
Position has to be a particular, amino acid can't be replaced
Conservatively Substituted
Position can be occupied by the same type of amino acid
Hypervariable
DNA segment that shows a high degree of variability between members of a population; only functions as a placeholder
Orthologous Proteins
Proteins from different species that have homologous amino acid sequences (and often a similar function.) They arose from a common ancestral gene during evolution.
Gene Duplication
The generation of extra copies of a gene in a genome over evolutionary time. A mechanism by which genomes can acquire new functions.
Paralogous Genes
Genes that are found in the same genome as a result of gene duplication but one has gained a new function.
A phylogenetic tree depicts ___________ of proteins.
A) folding patterns
B) hypervariable residues
C) invariable residues
D) evolutionary relationships
E) gene sequences
D) evolutionary relationships
A protein that has had few changes in its amino acid sequence over evolutionary history is labeled
A) a fibrinopeptide
B) evolutionarily conserved
C) random
D) a product of pseudogenes
E) phylogenetic
B) evolutionarily conserved
A fast way for nature to generate new proteins is:
A) generation of pseudogenes.
B) mutation by neutral drift.
C) shuffling protein domains or motifs.
D) hypervariable positions.
E) liberal substitution.
C) shuffling protein domains or motifs.
Proteins are often constructed from multiple segments of 40-200 amino acid residues, commonly called
A) pseudogenes
B) hypervariable residues
C) protolytic fragments
D) domains
E) subunits
D) domains
The amino acid sequence of which type of protein is likely to have changed the least during evolution?
A) A protein that makes no important interactions with other proteins
B) A protein fragment with no known function
C) A protein that has extensive interactions with other proteins and with DNA
D) A protein that has some interactions with other proteins
C) A protein that has extensive interactions with other proteins and with DNA
The _____ structure of a protein is the amino acid sequence of its polypeptide chain, or chains if the protein consists of more than one polypeptide.
Primary
True or False. Only a tiny fraction of all possible proteins exist (i.e. of all combinations of protein sequence possible, evolution has produced only a tiny fraction of the theoretical possibilities).
True
True or False. In an evolutionary time frame, the rate at which mutations are accepted into a protein depends upon the function of the protein.
True
Which one of the following statements about peptide bonds is FALSE. Peptide bonds are:
A) rigid and planar, with partial double-bond character
B) involved in forming the primary structure of proteins
C) charged
D) amides
E) covalent
C) charged
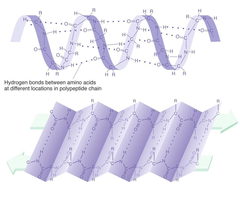
Which level of protein structure is defined as "the hydrogen bonded arrangement of the polypeptide backbone"?
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
B) Secondary
A rigid, planar structure between at least two amino acids consisting of about 40% double bond character is characteristic of a _______________.
Peptide group
Which of the following is FALSE with respect to β-sheets?
A) β-sheets may be parallel or anti-parallel.
B) Strands in a β-sheet are connected by irregular loops.
C) β-sheets are stabilized by non-covalent forces.
D) Amino acid side chains protrude from one side of the β-sheet
D) Amino acid side chains protrude from one side of the β-sheet
Repeating values of Φ and Ψ make up predictable orientations of amino acid with a chain, this predictable orientation forms _________________
Regular secondary structure
The strength of Entry field with _________ comes from close packing of glycine residues and the characteristics of hydroxyproline allowing formation of a left-handed helical conformation which combines with two other left-handed structures to form a right-handed triplet helix.
collagen
Helix-Entry field with _________ is the phenomenon in which side chains of residues just outside -helices make hydrogen bonds to the backbone groups of the four terminal residues of the helix.
capping
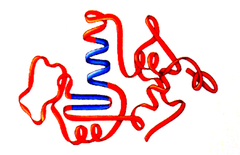
Which of the following describes the entire three-dimensional structure of a single polypeptide?
A) Tertiary structure
B) Secondary structure
C) Quaternary structure
D) Primary structure
A) Tertiary structure
What is the primary driving force in the formation of protein tertiary structure?
A) The exclusion of non-polar substances from aqueous solution.
B) The formation of van der Waals interactions between neighbouring groups.
C) Energy released when additional ion pairs are formed.
D) Energy released when additional hydrogen bonds are formed.
A) The exclusion of non-polar substances from aqueous solution.
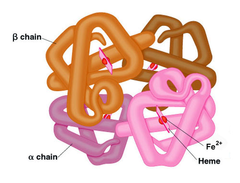
Which of the following statements about quaternary structure is TRUE?
A) Quaternary structure requires covalent interactions between polypeptide chains.
B) Quaternary structure is defined as the precise, 3-D arrangement of polypeptide backbones in proteins with more than one subunit.
C) Quaternary structure exists in monomeric proteins.
D) Quaternary structure is stabilized by the same types of noncovalent interactions as tertiary structure
D) Quaternary structure is stabilized by the same types of noncovalent interactions as tertiary structure
The overall arrangement of the regular structural elements such as the alpha helix and the β sheet in the protein are considered the protein's _________
tertiary structure
The __________ of an amino acid can be used to predict whether an amino acid side chain folds towards the inside or outside of a globular protein.
hydropathy
What is the major factor that "drives" the folding of proteins into their tertiary structure?
A) Placement of polar amino acid residues on the surface of the protein.
B) Placement of hydrophobic amino acid residues within the interior of the protein.
C) Formation of the maximum number of hydrophilic interactions.
D) Formation of the maximum number of ionic interactions.
B) Placement of hydrophobic amino acid residues within the interior of the protein.
Secondary Structure
The local spatial arrangement of a polypeptide's backbone atoms without regard for its side chain
Tertiary Structure
the three-dimensional form of an entire polypeptide, including its side chains
Quaternary Structure
the spatial arrangement of protein subunits
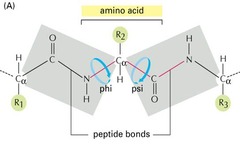
Ψ (Psi) Angle
angle in polypeptide backbone, torsion angle around the Cα-C bond
Φ (Phi) Angle
angle in polypeptide backbone, torsion angle around the Cα-N bond
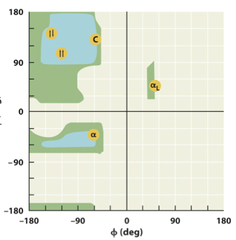
Ramachandran Diagram
A steric countour duagram that depicts allowed ranges of the angles phi and psi for amino acid residues in polypeptide cahins. For each residue, its confirmation in the main chain of polypeptide can be completely defined by the psi and phi angles
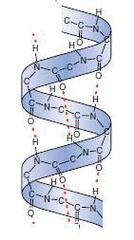
α-helix
A protein secondary structure - a right-handed spiral held in place by hydrogen bonds between adjacent C=O and NH groups
The side chains project outward and down which avoids steric hindrance
Tightly packed - van der Waals contact
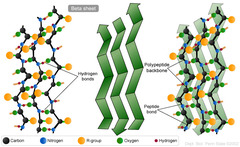
β-pleated sheet
An element of secondary structure, marked by peptide chains lying alongside one another, forming rows or strands
H-bonding occurs between polypeptide chains
Anti-parallel or parallel (slightly less stable)
Side chains extend to opposite sides of sheets
Globular Proteins
These proteins are small spheres with little to no water inside. They have hydrophobic amino acids in the inside and hydrophilic R groups on the outside
Water soluble, characterized by compact and highly folded structure
Fibrous Proteins
These form extended sheets or strands, making them tough, durable, and generally insoluble in water; form fibers
Keratin
fibrous protein, mechanically durable and relatively unreactive, two alpha helices coiling around each other, heptad repeat
Lots of keratin causes curly hair